Herpesvirus-Vectored Transmissible Vaccines Show Promise
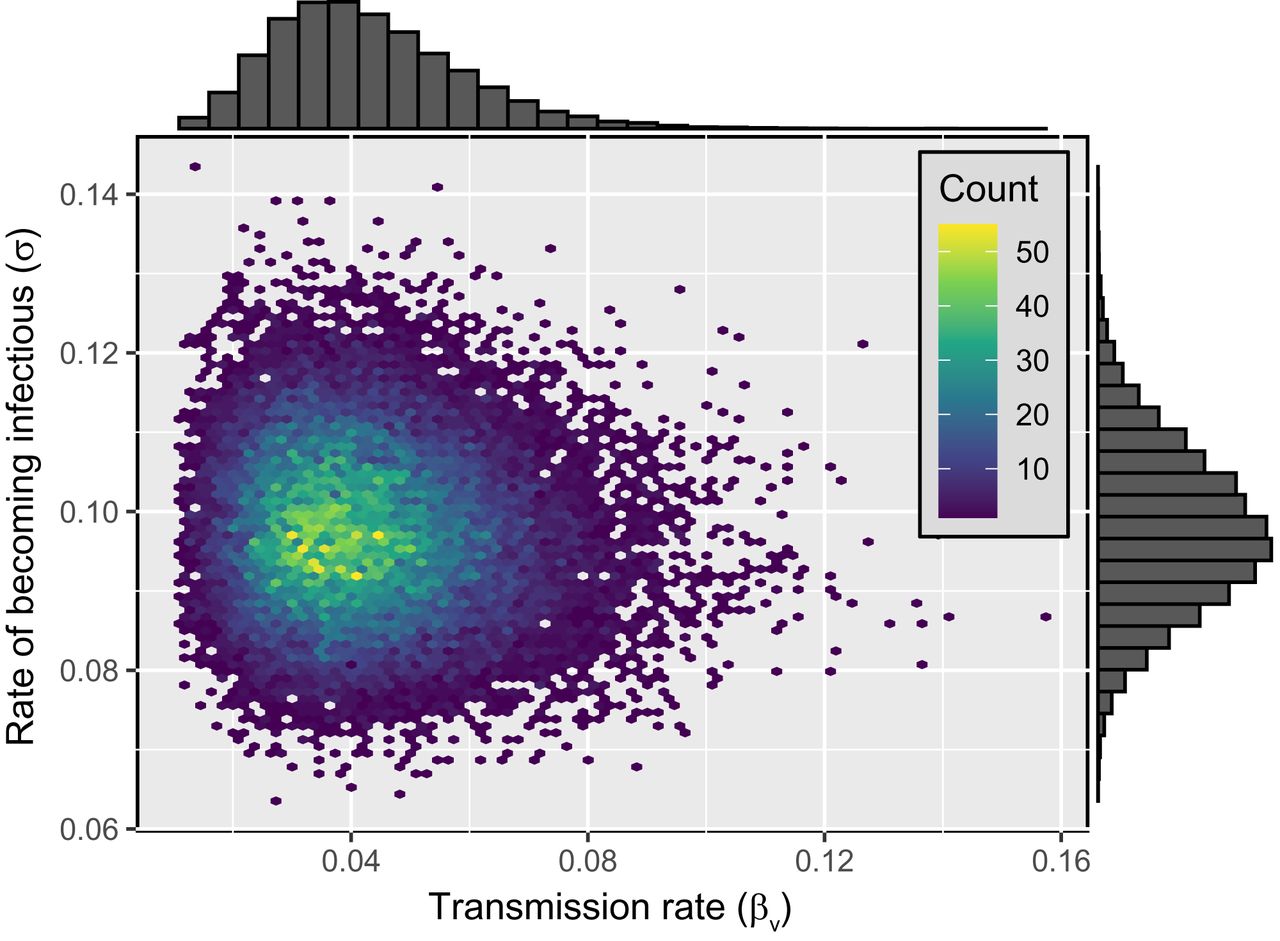
January 20, 2022Bivariate posterior distribution of the transmission rate (βv) and the rate at
which individuals exposed to the virus transition into the infectious class (σ).
The spillover of infectious diseases from animals to humans is becoming an increasing threat to our health and welfare. Transmissible vaccines have the potential to revolutionize how zoonotic pathogens are controlled in wildlife populations and prevent the spread of these diseases to humans.
Recent graduate of the Bioinformatics and Computational Biology doctoral program Tanner Varrelman published a paper in PNAS examining the effectiveness of herpesviruses as vectors for transmissible vaccines. He found that these types of vaccines could have the potential to manage infectious diseases in wildlife populations.
Most current approaches to managing zoonotic pathogens are reactive, and the delay between emergence and control of a disease can have deadly consequences. Using mathematical models, Varrelman and his co-authors evaluated a more proactive approach to managing emerging infectious diseases and eliminating pathogens from wild animal populations before spillover can occur.
The authors focused on herpesviruses as a vector, or carrier, for the vaccine because of their ability to rapidly spread immunity throughout a population. Betaherpesviruses are also species-specific and stable to genetic mutation, making them prime candidates for transmissible vaccines vectors.
Varrelman demonstrated that these types of vaccines may have the potential to rapidly and effectively control a range of zoonotic pathogens in wild animals. However, the results also suggest that the effectiveness of the vaccine varies depending on the population and the specific vector strain used to construct it.
Ultimately, transmissible vaccines show promise for mitigating the risk of zoonotic diseases but require more extensive research on the interactions between vector strain diversity and infection in the wild.